|
|
| 靶向BCMA的CAR-T细胞治疗多发性骨髓瘤的I期临床研究 | BMC Journal |
|
论文标题:A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma
期刊:Journal of Hematology & Oncology
作者:Wan-Hong Zhao†, Jie Liu†, Bai-Yan Wang†, Yin-Xia Chen, Xing-Mei Cao, Yun Yang, Yi-Lin Zhang, Fang-Xia Wang, Peng-Yu Zhang, Bo Lei, Liu-Fang Gu, Jian-Li Wang, Nan Yang, Ru Zhang, Hui Zhang, Ying Shen, Ju Bai, Yan Xu, Xu-Geng Wang, Rui-Li Zhang, Li-Li Wei, Zong-Fang Li, Zhen-Zhen Li, Yan Geng, Qian He, Qiu-Chuan Zhuang, Xiao-Hu Fan, Ai-Li He and Wang-Gang Zhang
发表时间:2018/12/20
数字识别码:10.1186/s13045-018-0681-6
原文链接:http://t.cn/AiOwU3NU
微信链接:https://mp.weixin.qq.com/s/xcDfoyJfzQSrLTbnfJeudA
多发性骨髓瘤是一种浆细胞来源的恶性肿瘤,占血液系统恶性肿瘤的13%,绝大多数患者终会面临复发,至今仍无法治愈,预后较差。B细胞成熟抗原(BCMA)是肿瘤坏死因子超家族成员,广泛表达于骨髓瘤细胞表面,但在正常细胞表达很低,在CD34阳性的造血细胞中不表达,因此是一个理想的靶标。
嵌合抗原受体T细胞(CAR-T)治疗在血液系统肿瘤中已被证明其卓越的疗效,西安交通大学第二附属医院血液内科团队与南京传奇生物科技有限公司合作,自2016年开始,开展了一项靶向B细胞成熟抗原(BCMA)的CAR-T细胞(LCAR-B38M)治疗难治/复发多发性骨髓瘤的1期开放性临床研究。研究结果于2018年12月发表在开放获取期刊Journal of Hematology & Oncology 上。
这项临床研究主要目的是评估针对BCMA的双表位结合CAR-T细胞疗法——LCAR-B38M的安全性及有效性。在这项1期、单臂、开放标签的多中心临床研究中,共纳入了74例难治复发的多发性骨髓瘤患者,该文章介绍了西安交通大学第二附属医院该中心的57例患者的研究结果。

该研究招募年龄在18-80岁的难治/复发多发性骨髓瘤患者(R/R MM),经过环磷酰胺预处理(300mg/m2•d,在-5,-4,-3天给予)进行淋巴细胞清除后,分三次输注LCAR-B38M细胞(7天内分别输注总剂量的20%,30%及50%)。主要研究目标为评价LCAR-B38M的安全性,次要目标为评价其抗骨髓瘤的有效性(以IMWG评估标准)。
截止随访数据时,共有57例难治/复发多发性骨髓瘤患者接受了LCAR-B38M CAR-T细胞输注,中位年龄为54岁(27-72岁),ECOG评分0-2级,中位起病时间为4年(1-9年),中位既往治疗线数为3线(1-9线),其中18%的患者接受过自体造血干细胞移植,68%的患者应用过蛋白酶体抑制剂,86%的患者应用过免疫调节剂,两种药物均用过者占60%。中位输注CAR-T细胞数为0.5×106细胞/kg (范围:0.07-2.1×106),中位随访时间为8个月(范围:0.7-20.7月)。
所有患者至少经历有1项不良事件(AEs),发生大于3级AEs患者为37例(65%),其中最常见的不良事件为白细胞减低(17/57;30%)、血小板减少(13/57 ;23%)、谷草转氨酶的升高(12/57;21%)。51例(90%)患者发生了细胞因子释放综合征(CRS),主要为1级(47%)和2级(35%),≥3级者有4例患者(7%),其中1例患者有表现为1级的失语、焦虑及癫痫样表现的神经毒性。CRS的严重程度与白细胞介素6(IL-6)升高的峰值相关,其他细胞因子还包括IL-2、IL-8、IL-10及肿瘤坏死因子α(TNFα)。
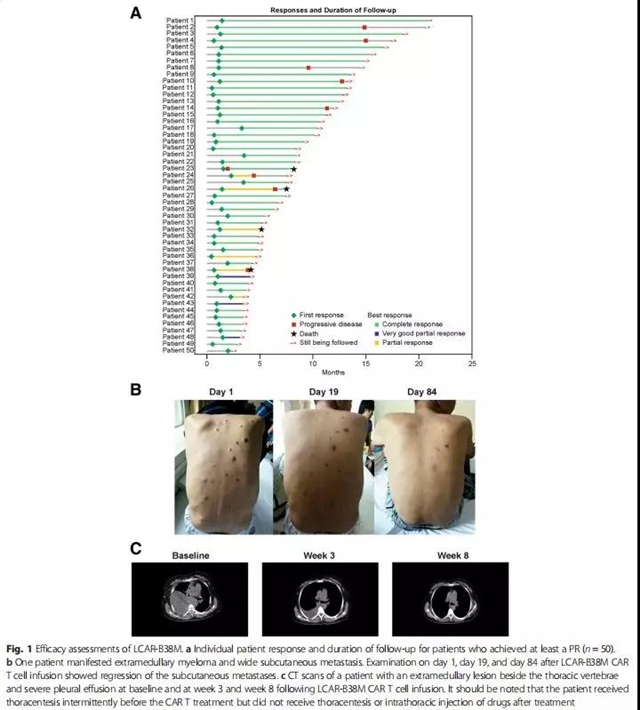
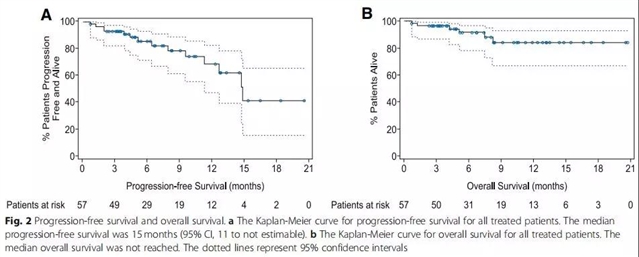
治疗总反应率(ORR)为88%(95% CI,76-95),其中取得完全缓解(CR)、非常好的部分缓解(VGPR)及部分缓解(PR)的患者分别占68%(n=39)、5%(n=3)及14%(n=8)。其中取得CR的患者63%(n=36)经8色流式细胞术检测骨髓微小残留病灶阴性。中位持续反应时间为14个月(95% CI,12-不可估),中位无进展生存为15个月(95% CI,11-不可估),中位总生存还未达到。
这项首次应用于患者的双表位结合的LCAR-B38M CAR-T细胞治疗的临床试验初步证明,这是一种对难治/复发多发性骨髓瘤患者非常有效的治疗方法,LCAR-B38M显示了与其已知作用机制一致的安全可控性,是一种很有前途和潜力的治疗方法。进一步的在临床实验正在进行中,其中美国已经开始了1b/2阶段的临床研究(ClinicalTrials.gov, NCT03548207)。
摘要:
Background
Chimeric antigen receptor (CAR) T cell therapy has demonstrated proven efficacy in some hematologic cancers. We evaluated the safety and efficacy of LCAR-B38M, a dual epitope-binding CAR T cell therapy directed against 2 distinct B cell maturation antigen epitopes, in patients with relapsed/refractory (R/R) multiple myeloma (MM).
Methods
This ongoing phase 1, single-arm, open-label, multicenter study enrolled patients (18 to 80 years) with R/R MM. Lymphodepletion was performed using cyclophosphamide 300 mg/m2. LCAR-B38M CAR T cells (median CAR+ T cells, 0.5 × 106 cells/kg [range, 0.07 to 2.1 × 106]) were infused in 3 separate infusions. The primary objective is to evaluate the safety of LCAR-B38M CAR T cells; the secondary objective is to evaluate the antimyeloma response of the treatment based on the general guidelines of the International Myeloma Working Group.
Results
At data cutoff, 57 patients had received LCAR-B38M CAR T cells. All patients experienced ≥ 1 adverse events (AEs). Grade ≥ 3 AEs were reported in 37/57 patients (65%); most common were leukopenia (17/57; 30%), thrombocytopenia (13/57; 23%), and aspartate aminotransferase increased (12/57; 21%). Cytokine release syndrome occurred in 51/57 patients (90%); 4/57 (7%) had grade ≥ 3 cases. One patient reported neurotoxicity of grade 1 aphasia, agitation, and seizure-like activity. The overall response rate was 88% (95% confidence interval [CI], 76 to 95); 39/57 patients (68%) achieved a complete response, 3/57 (5%) achieved a very good partial response, and 8/57 (14%) achieved a partial response. Minimal residual disease was negative for 36/57 (63%) patients. The median time to response was 1 month (range, 0.4 to 3.5). At a median follow-up of 8 months, median progression-free survival was 15 months (95% CI, 11 to not estimable). Median overall survival for all patients was not reached.
Conclusions
LCAR-B38M CAR T cell therapy displayed a manageable safety profile and demonstrated deep and durable responses in patients with R/R MM.
Trial registration
ClinicalTrials.gov, NCT03090659; Registered on March 27, 2017, retrospectively registered
阅读论文全文请访问:
http://t.cn/AiOwU3NU
期刊介绍:
Journal of Hematology & Oncology(https://jhoonline.biomedcentral.com/, 8.731 -2-year Impact Factor,7.373 -5-year Impact Factor) is an open access journal that publishes top-quality research encompassing all aspects of hematology and oncology. The journal also publishes reviews and research highlights on "hot topics" from leading experts in the field.
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。