|
|
| MDPI Vaccines | 米替福辛作为流感疫苗佐剂的再利用 |
|
论文标题:Repurposing of Miltefosine as an Adjuvant for Influenza Vaccine
(米替福辛作为流感疫苗佐剂的再利用 )
期刊:Vaccines
作者:Lu Lu, Carol Ho-Yan Fong,Anna Jinxia Zhang,Wai-Lan Wu,Iris Can Li,Andrew Chak-Yiu Lee,Thrimendra Kaushika Dissanayake,Linlei Chen,Ivan Fan-Ngai Hung,Kwok-Hung Chan,Hin Chu, Kin-Hang Kok,Kwok-Yung Yuen and Kelvin Kai-Wang To
发表时间:11 December 2020
DOI:10.3390/vaccines8040754
微信链接:
https://mp.weixin.qq.com/s?__biz=MzI1MzEzNjgxMQ==&mid=2649979572&idx=2&sn=
8d9d25e4fc5537c1abaa3cac99fbe7e7&chksm=f1dec070c6a94966d29c160c36b8ebc2f
3b4ba0915428650ec34cd3240617e4e9d371627f618&token=287653195&lang=zh_CN#rd
期刊链接:https://www.mdpi.com/journal/vaccines
大流行性流感、季节性流感都有着很高的发病率和死亡率,研究证明疫苗在预防流感方面发挥着至关重要的作用,而且流感疫苗能显著减少并发症。但是目前获批的流感疫苗仍然存在一些问题:
流感疫苗的效力还不是很高,目前来说获批的流感疫苗总效力大约只有60%;
当前的流感疫苗对年龄较大或者身体免疫力低的人群,有效性较低;
当前的流感疫苗只有在接种2-3周后才能使人体产生足够的抗体。
因此,寻找一种能增强流感疫苗效力、减短疫苗起效时间的疫苗佐剂显得尤为重要。米替福辛 (Miltefosine, MFT) 是经美国食品和药物管理局 (Food and Drug Administration, FDA) 批准的一种疫苗佐剂。如果证明MFT用于流感疫苗是可行的,则意味着可以大大缩短疫苗用于流感疫苗临床阶段试验的时间。
近期,来自香港大学的Kelvin Kai-Wang To博士及其团队发表在Vaccines期刊上的文章,评估了MFT作为潜在流感疫苗佐剂的可能性,并描述了其作用机制。
实验过程
对照组 (VAC组)
对小鼠注射不含MET佐剂的流感疫苗 (vaccine-only, VAC)
实验组 (MTF-VAC组)
对小鼠注射含有MET佐剂的流感疫苗 (Miltefosine-vaccine, MTF-VAC)
MTF佐剂在7天内可提高疫苗效力
为了确定MTF的佐剂效果,分别用有或没有MTF的流感疫苗对小鼠进行接种,并在接种7天后用流感病毒415742Md进行病毒攻击。然后在攻击后的第7天,对注射不同含量MTF流感疫苗和空白对照组小鼠的感染情况进行检测。实验发现,接种了0.08 mg MFT的MTF-VAC组小鼠的存活率、体重、肺损伤情况以及病毒载量明显优于其他组小鼠 (如图1)。
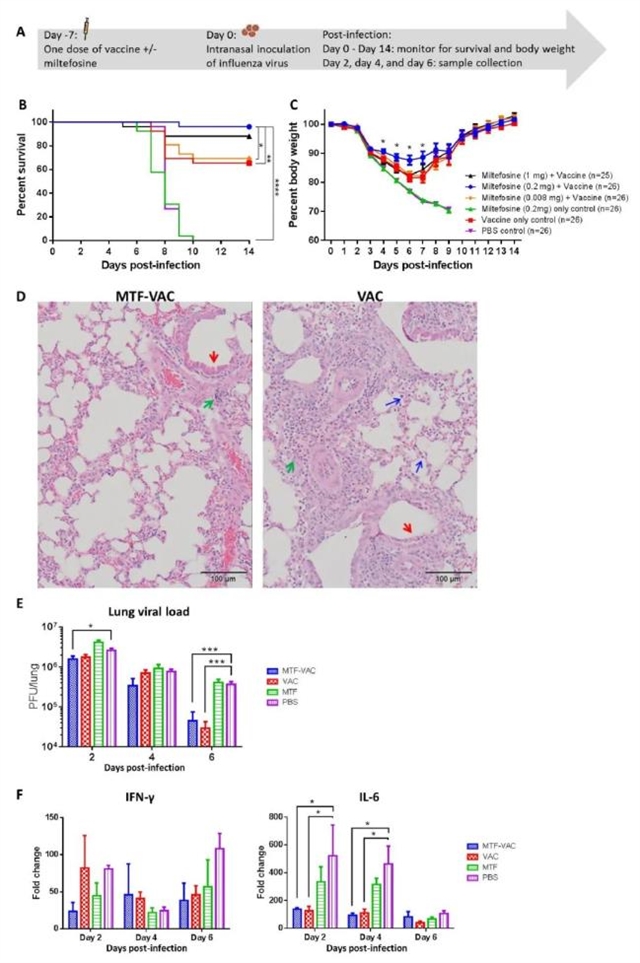
图1. 在流感病毒攻击前7天内MTF对小鼠生存率、体重、肺损伤情况以及病毒载量的影响。
MTF佐剂可增强和扩大流感疫苗的抗体应答
小鼠接种疫苗7天后对其进行病毒攻击,在攻击后第4和6天时评估小鼠的体内抗体反应。实验发现,接种MTF佐剂疫苗的小鼠体内免疫球蛋白水平明显高于对照组。接种MTF佐剂疫苗组比其他组诱导了更多的抗体反应。为了评估MTF是否能提高抗抗原漂移病毒的抗体应答,作者对小鼠感染后收集的血清样本进行了抗抗原漂移2018 H1N1病毒的检测。在接种第6天采集的小鼠血清标本中,实验组对2018年H1N1病毒的抗体滴度显著高于对照组 (p < 0.001) (图2)。
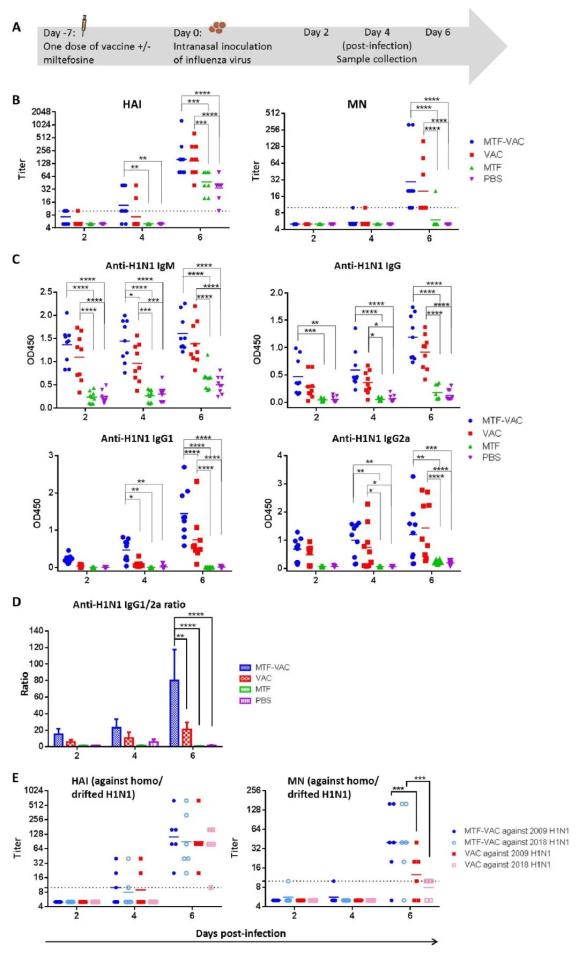
图2. 病毒攻击前7天MTF对小鼠体内抗体滴度的影响。
MTF佐剂可降低疫苗的起效时间
为了评估这种疫苗是否能降低起效时间,作者对接种3天疫苗的小鼠进行病毒攻击,比较各组小鼠的存活率、体重、体内抗体反应水平、肺损伤情况和病毒载量。实验组生存率 (80%) 显著高于对照组 (26.7%)。注射0.2 mg MTF的小鼠存活率高于注射0.008 mg MTF的小鼠 (53.3%),但其差异无统计学意义 (p = 0.1607)。两组小鼠的体重下降无显著性差异。实验发现MTF佐剂可降低疫苗的起效时间,在3天内提高疫苗疗效 (如图3)。
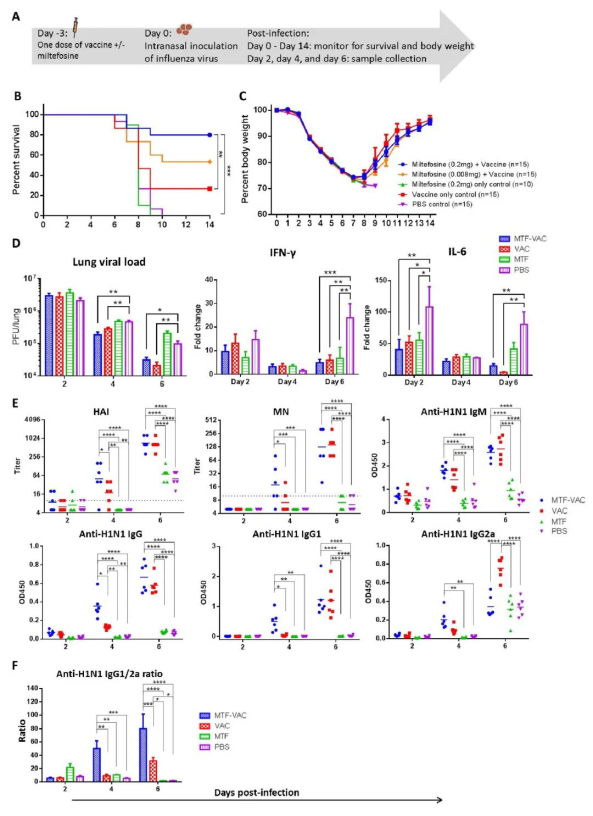
图3,病毒攻击前3天MTF对小鼠的影响。
在流感病毒感染前3天或7天,小鼠的基本情况如下:
MTF-VAC组的存活率明显优于VAC组;
MTF-VAC组肺损伤明显改善;
MTF-VAC组对同源和异源流感毒株的抗体反应均显著增强;
MTF-VAC组的小鼠产生了较多的T滤泡辅助细胞 (T follicular helper cell, TFH) 反应和增强的淋巴生发中心反应;
MTF-VAC可诱导1型T细胞和2型T细胞抗原特异性细胞因子反应;
MTF可通过提高TFH和抗体反应,提高流感疫苗对同源和异种病毒的效力。
局限性
第一,在本研究中,作者证明了MFT能增强淋巴细胞诱导的抗体反应。然而,其他不涉及淋巴细胞的途径也可能起作用。例如,MTF可以直接刺激B细胞向浆细胞分化。第二,本研究的实验对象是6 - 8周龄的小鼠,MTF在老年人和免疫缺陷患者人群中是否有效仍有待确定。还需要进一步用小鼠的异种病毒攻毒试验来验证MTF-VAC的交叉保护作用。此外,MTF抗原呈递细胞的影响有待进一步阐明,特别是有文献报道米替福辛可刺激抗原呈递细胞 (如树突状细胞和巨噬细胞上的Toll样受体4和Toll样受体9)。第三,作者是通过腹腔注射米替福辛和流感疫苗,而不是人类使用的肌肉或皮内注射途径。
实验结论
经实验发现,FDA批准的MTF是一种有效的疫苗佐剂。由于该药物的全身和局部安全性已经为人所知,MTF作为疫苗佐剂可以比其他未获批准的佐剂更快地在临床试验中进行评估。作者的研究表明了MTF在提高疫苗诱导免疫原性方面的重要作用。结果显示米替福辛不仅可以用于流感疫苗,也可用于其他疫苗的评估,包括即将推出的COVID-19疫苗。
Vaccines(ISSN 2076-393X, IF 4.086) 是一个国际型开放获取期刊。期刊主题涵盖疫苗,抗体,免疫等方面。Vaccines采取单盲同行评审,一审周期约为13.6天,文章从接收到发表仅需3.4天。
Background
We previously reported that topical imiquimod can improve the immunogenicity of the influenza vaccine. This study investigated another FDA-approved drug, miltefosine (MTF), as a vaccine adjuvant. Mice immunized with an influenza vaccine with or without MTF adjuvant were challenged by a lethal dose of influenza virus 3 or 7 days after vaccination. Survival, body weight, antibody response, histopathological changes, viral loads, cytokine levels, and T cell frequencies were compared. The MTF-adjuvanted vaccine (MTF-VAC) group had a significantly better survival rate than the vaccine-only (VAC) group, when administered 3 days (80% vs. 26.7%, p = 0.0063) or 7 days (96% vs. 65%, p = 0.0041) before influenza virus challenge. Lung damage was significantly ameliorated in the MTF-VAC group. Antibody response was significantly augmented in the MTF-VAC group against both homologous and heterologous influenza strains. There was a greater T follicular helper cell (TFH) response and an enhanced germinal center (GC) reaction in the MTF-VAC group. MTF-VAC also induced both TH1 and TH2 antigen-specific cytokine responses. MTF improved the efficacy of the influenza vaccine against homologous and heterologous viruses by improving the TFH and antibody responses. Miltefosine may also be used for other vaccines, including the upcoming vaccines for COVID-19.
Method
Data in this project are displayed as means or geometric means with the standard error of mean (SEM). For survival comparison, log–rank (Mantel–Cox) test was used. For body weight comparisons, multiple t-tests were applied. For pulmonary viral load, the statistical comparisons were conducted on logarithmized viral load with multiple t-tests. Relative expression levels of cytokine genes in each mouse were normalized by β-actin expression level and fold changes were calculated by comparing to naïve mice. Statistical comparisons of fold changes were conducted by two-way ANOVA. All statistical comparisons of antibody titers were conducted by two-way ANOVA. For HAI and MN titers, the undetectable samples were taken as titers of 5 for calculation. The titers were logarithmized before comparison. Frequencies of TFH cells, frequencies of GCs, and numbers of antigen-specific cytokine secreting cells were compared by two-way ANOVA. Asterisk markers for significance levels: * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. All comparisons were conducted in GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
The effectiveness of the influenza vaccine is suboptimal. Vaccine adjuvant has been used in boosting the immune response. In this study, we have found that MTF, an FDA-approved anti-leishmaniasis drug, significantly improved the protective efficacy of influenza vaccines. After the lethal challenge of A(H1N1) virus, the MTF-VAC group had a significantly higher survival rate than the VAC group. The improved survival in the adjuvanted group occurred as soon as 3 days after vaccination, and was accompanied by an accelerated and augmented antibody response, and a more robust TFH cell response. Both TH1 and TH2 responses were stimulated. Furthermore, we have demonstrated that the MTF-adjuvanted vaccine induced a better antibody response against antigenically-drifted influenza viruses within the same subtype.
Conclusions
Our study showed that the FDA-approved MTF is a potent vaccine adjuvant. Since the systemic and topical safety profile of this drug is already known, MTF as a vaccine adjuvant can be assessed in clinical trials much sooner than other non-approved adjuvants. Our study highlighted the important role of TFH in improving vaccine-induced immunogenicity. The results of this study go beyond the influenza vaccine. Miltefosine can also be evaluated for other vaccines, including the upcoming COVID-19 vaccines.


(来源|:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。