|
|
|
|
|
肝脏部分切除后细胞周期再进入同步化的新机制 | Cell Regeneration |
|
|
论文标题:Capn3 depletion causes Chk1 and Wee1 accumulation and disrupts synchronization of cell cycle reentry during liver regeneration after partial hepatectomy
期刊: Cell Regeneration
作者:Feng Chen, Delai Huang et.al
发表时间:2020/06/11
数字识别码:10.1186/s13619-020-00049-1
微信链接:点击此处阅读微信文章
肝切除术是目前治疗各种肝脏良恶性疾病的有效方法之一,肝切除后,肝脏内剩余的实质细胞及各种细胞因子、生长因子会迅速做出反应,启动肝脏再生能力,在短时间内即可恢复肝脏正常体积和肝功能。但是如果肝脏再生出现障碍,极易引起肝衰竭,最终导致患者死亡。如何快速地启动肝脏再生机制,仍然是目前的研究热点之一。
过去研究表明,肝脏部分切除之后,剩余肝脏细胞增殖过程受细胞周期影响,从G0期向G1期过渡。所有细胞进入同步化的细胞周期,DNA复制几乎发生在同一个时间点。但为什么在切除后的肝脏组织中,细胞周期会重新进入同步化的调控机制并不清楚。
2020年6月,来自浙江大学的彭金荣团队在Cell Regeneration上发表了题为“Capn3 depletion causes Chk1 and Wee1 accumulation and disrupts synchronization of cell cycle reentry during liver regeneration after partial hepatectomy”的论文,报道了一个新的蛋白对细胞周期重新进入同步化的调控作用。

图1
该研究主要关注半胱氨酸钙蛋白酶3(Capn3,在斑马鱼中该基因被命名为Capn3b),并制备了敲除Capn3b的斑马鱼模型。虽然敲除Capn3b的斑马鱼可以稳定生存和繁殖,但在一些应激状态比如不同密度、不同温度的饲养条件下,敲除Capn3b的胚胎发育是受影响的,表现为身体呈弧形、生长迟缓、肝体比(用于评估肝脏再生的指标)比例失调 (Figure 2)。
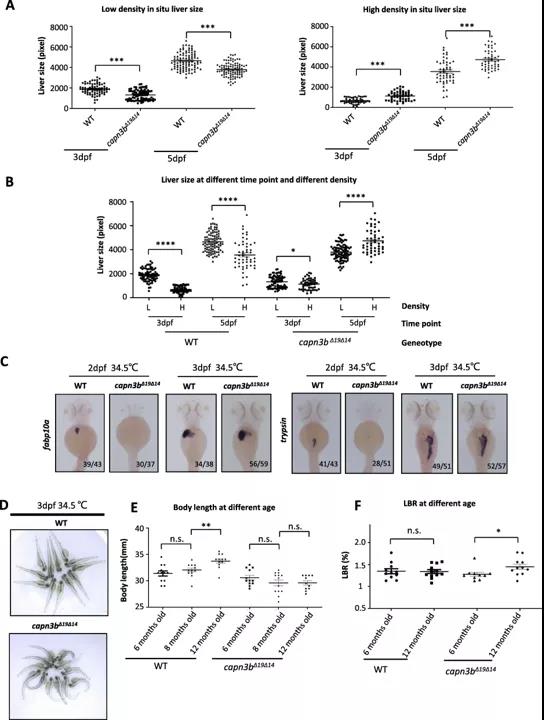
图2
当将Capn3b敲除斑马鱼肝脏腹侧叶摘除后,一开始其肝脏再生速度远远低于野生型,无论是切除区域和深层区域的细胞增殖能力均远远弱于野生型;而当野生型肝脏再生基本完成时,同时期的Capn3b敲除肝脏切除区域的细胞才开始启动再生机制,而深层区域的细胞DNA复制水平仍然很低。这说明在Capn3b敲除的肝脏细胞中,细胞周期再进入不仅延迟,而且并未同步化(Figure 3C-F,Figure 4)。
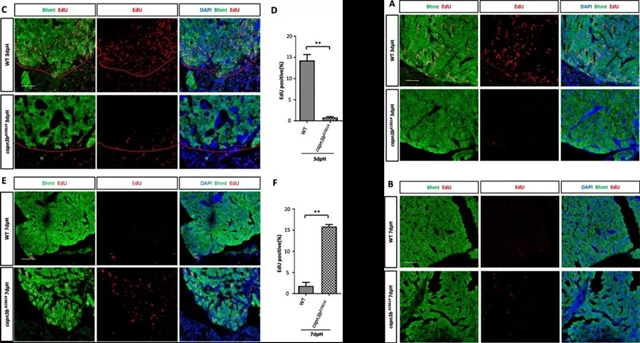
图3
接下来,作者提取了野生型未处理、野生型肝脏腹侧叶摘除、Capn3b敲除型未处理、Capn3b敲除型肝脏腹侧叶摘除这四组肝脏细胞的核蛋白,并进行了质谱分析,找到了许多与核仁RNA功能相关的差异蛋白。除此之外,作者鉴定出G2-M检查点蛋白激素Wee1和上游调节因子Chk1均存在与Capn3b存在相互作用的区域,且这些相互作用区域从斑马鱼到人是高度保守的。初步推断Wee1和Chk1与Capn3b在核仁中能够形成复合体。随后进一步发现,当肝叶被摘除后,Wee1和Chk1在Capn3b敲除型肝脏组织中大量堆积。这可能是Capn3b敲除引起肝再生延迟的原因之一(Figure 8E-F,Figure 9)。
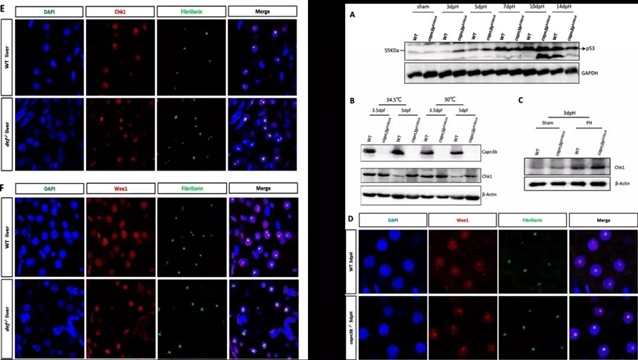
图4
综上,该研究揭示了核定位的defi - capn3复合物能够通过裂解两种明确的细胞周期负性调节因子Chk1和Wee1在肝切除后的肝脏再生过程中发挥作用,进一步阐明了肝脏再生的分子机理。
彭金荣课题组博士陈峰博士为本文第一作者,中国科学院遗传与发育生物学研究所的汪迎春研究员和浙江大学的彭金荣教授为共同通讯作者。该研究得到了国家自然科学基金和国家重点研发计划的经费支持。

图5
Cell Regeneration 是开放获取期刊,目前所有的稿件处理费用(APC)将由中国细胞生物学学会负责,作者无需支付任何费用。
期刊网址:cellregeneration.springeropen.com ,欢迎点击“阅读原文”了解更多Cell Regeneration 期刊详情,我们期待您的投稿。
Cell Regeneration 首批文章上线(点击阅读详情)
摘要:Recovery of liver mass to a healthy liver donor by compensatory regeneration after partial hepatectomy (PH) is a prerequisite for liver transplantation. Synchronized cell cycle reentry of the existing hepatocytes after PH is seemingly a hallmark of liver compensatory regeneration. Although the molecular control of the PH-triggered cell cycle reentry has been extensively studied, little is known about how the synchronization is achieved after PH. The nucleolus-localized protein cleavage complex formed by the nucleolar protein Digestive-organ expansion factor (Def) and cysteine proteinase Calpain 3 (Capn3) has been implicated to control wounding healing during liver regeneration through selectively cleaving the tumor suppressor p53 in the nucleolus. However, whether the Def-Capn3 complex participates in regulating the synchronization of cell cycle reentry after PH is unknown. In this report, we generated a zebrafish capn3b null mutant (capn3b?19?14). The homozygous mutant was viable and fertile, but suffered from a delayed liver regeneration after PH. Delayed liver regeneration in capn3b?19?14was due to disruption of synchronized cell proliferation after PH. Mass spectrometry (MS) analysis of nuclear proteins revealed that a number of negative regulators of cell cycle are accumulated in the capn3b?19?14 liver after PH. Moreover, we demonstrated that Check-point kinase 1 (Chk1) and Wee1, two key negative regulators of G2 to M transition, are substrates of Capn3. We also demonstrated that Chk1 and Wee1 were abnormally accumulated in the nucleoli of amputated capn3b?19?14 liver.In conclusion, our findings suggest that the nucleolar-localized Def-Capn3 complex acts as a novel regulatory pathway for the synchronization of cell cycle reentry, at least partially, through inactivating Chk1 and Wee1 during liver regeneration after PH.
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。